- Continuous Manufacturing
- Projects and Cases
Our know-how for your production
Projects & Case Studies
Our specialists from R&D, engineering and sales are available to our customers, especially when developing new production processes or changing from a previously used batch process to continuous manufacturing. Our goal is to realize the best result for the continuous manufacturing of tablets together with the customer.
On the way to the introduction of Continuous Manufacturing in the pharmaceutical industry, we accompany our customers from the first planning idea to the installation of the system at the production site. Even after that, we check and configure all required parameters for you. Even after that, we check and configure all required parameters for you.
With our comprehensive product range for Continuous Manufacturing for pharmaceutical tablet production, we enable continuous production across all established production processes. The following practical examples provide an initial overview of successfully implemented projects.

L.B. Bohle
Projects
Continuous Manufacturing
QbCon® 1 for a University in the United States

QbCon® 1 is already being used successfully by numerous producers in practice and above all in research and development. For the first time, QbCon® 1 enables a truly continuous granulation and drying process. Our patented fluid bed drying guarantees the material flow from the raw material to the dried granules in closed continuous operation.
At the university in the USA, QbCon® 1 is used for scientific studies, e.g. on the residence time of the granules or for process optimization.”
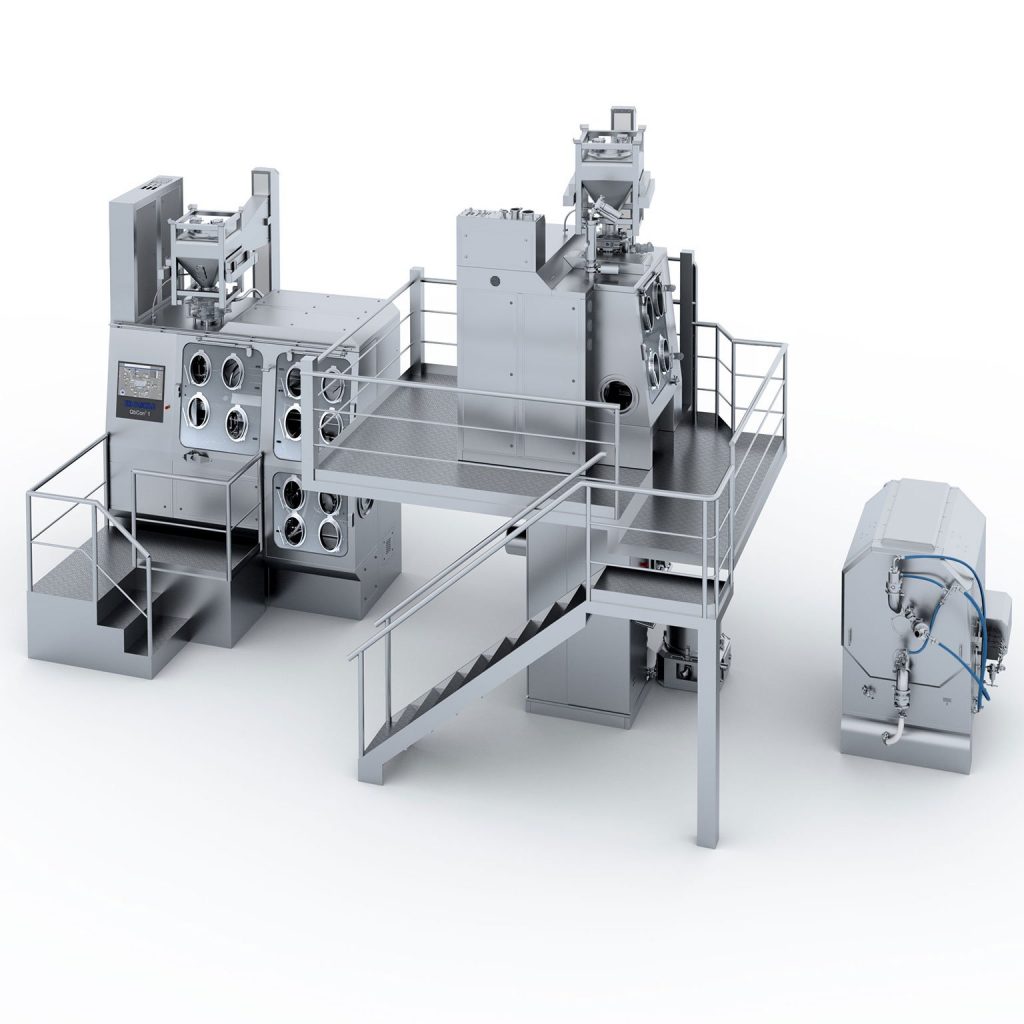
QbCon® WG for Generics Manufacturer

QbCon® – High-Containment
KOCO® - Semi-Continuous Tablet Coater
At a site in Europe, an international pharmaceutical company produces tablets on a KOCO® 25 in continuous operation.
Several products will be produced on the flexible tablet coater, which ensures high throughput, best coating results and uniformity.

Your contact for
Continuous Manufacturing
Burkhard Schmidt
Sales Director
inquiry@lbbohle.de
+49 2524 – 93 23 0

